Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19)
Key Points
- This guidance is intended for clinical laboratory and support staff who handle or process specimens associated with COVID-19. For guidance on point-of-rare testing, see the Guidance for SARS-CoV-2 Point-of-Care and Rapid Testing
- All laboratories should perform a site-specific and activity-specific risk assessment and follow Standard Precautions when handling clinical specimens. See Biological Risk Assessment: General Considerations for Laboratories
- Refer to List Non the Environmental Protection Agency (EPA) website for EPA-registered disinfectants that have qualified under EPA’s emerging viral pathogens program for use against SARS-CoV-2.
- Cultures of SARS-CoV-2 should be handled in a Biosafety Level 3 (BSL-3) laboratory using BSL-3 practices, and inoculation of animals with infectious wild-type SARS-CoV-2 should be conducted in an Animal Biosafety Level 3 (ABSL-3) facility using ABSL-3 practices and respiratory protection.
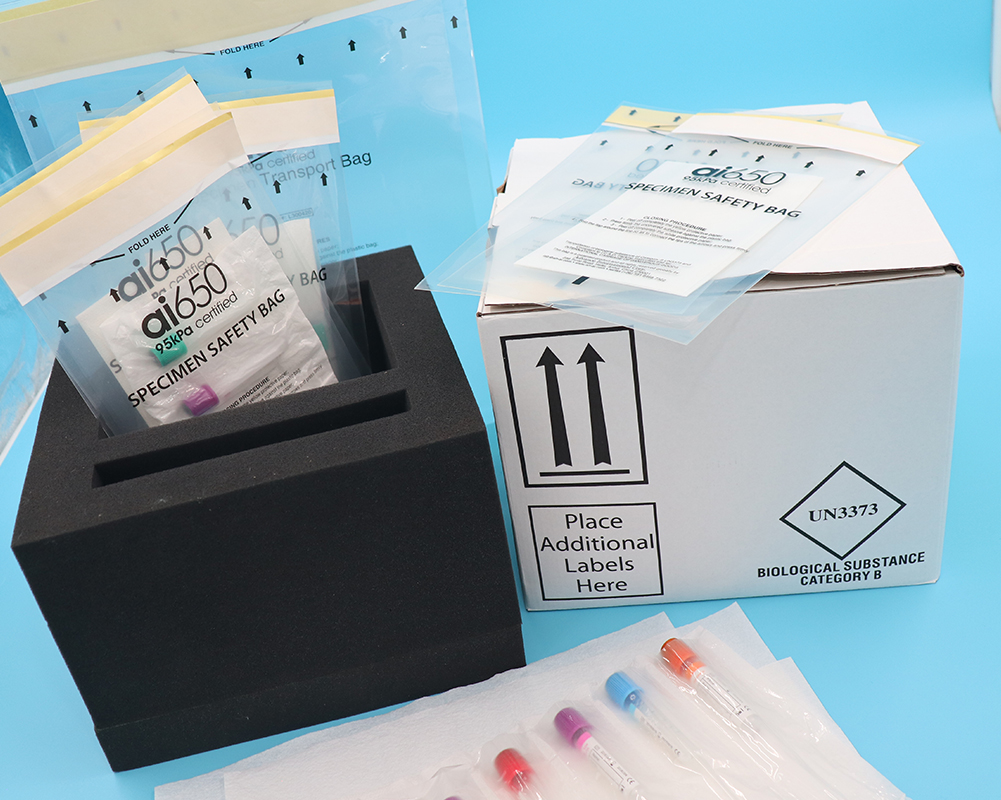
- Suspected and confirmed SARS-CoV-2 positive clinical specimens, cultures, or isolates should be packed and shipped as UN 3373 Biological Substance, Category B.
All clinical specimens may contain potentially infectious materials.
Precautions should be taken when handling specimens suspected or confirmed to be positive for SARS-CoV-2, the virus that causes COVID-19.
Timely communication between clinical and laboratory staff is essential to minimize the potential risk of handling specimens from patients with possible SARS-CoV-2 infection.
These specimens should be labeled accordingly, and the receiving laboratory should be alerted to ensure proper specimen handling.
General and specific biosafety guidelines for handling SARS-CoV-2 specimens are provided below.
General Guidance
All laboratories should perform a site-specific and activity-specific risk assessment to identify and mitigate risks. Risk assessments and mitigation measures are dependent on:
- The procedures performed
- Identification of the hazards involved in the process and procedures
- The competency level of the personnel who perform the procedures
- The laboratory equipment and facility
- The resources available
Follow Standard Precautions | Section IV when handling clinical specimens, all of which may contain infectious materials. Standard Precautions include hand hygiene and the use of specific personal protective equipment (PPE) determined by the potential for exposure to blood, body fluids, and infectious material. PPE, such as laboratory coats or gowns, gloves, eye protection, or a disposable mask and face shield, can help protect the skin and mucous membranes of the eyes, nose, and mouth.

No responses yet